Doxycycline Malaria Cost
2016年10月22日
Malaria
Quartan malaria; Falciparum malaria; Biduoterian fever; Blackwater fever; Tertian malaria; Plasmodium
Back to Top Causes
Malaria is caused by a parasite that is passed from one human to another by the bite of infected Anopheles mosquitoes. After infection, the parasites (called sporozoites) travel through the bloodstream to the liver, where they mature and release another form, the merozoites. The parasites enter the bloodstream and infect red blood cells.
The parasites multiply inside the red blood cells, which then break open within 48 to 72 hours, infecting more red blood cells. The first symptoms usually occur 10 days to 4 weeks after infection, though they can appear as early as 8 days or as long as a year after infection. The symptoms occur in cycles of 48 to 72 hours.
Most symptoms are caused by:
- The release of merozoites into the bloodstream
- Anemia resulting from the destruction of the red blood cells
- Large amounts of free hemoglobin being released into circulation after red blood cells break open
Malaria can also be transmitted from a mother to her unborn baby (congenitally) and by blood transfusions. Malaria can be carried by mosquitoes in temperate climates, but the parasite disappears over the winter.
The disease is a major health problem in much of the tropics and subtropics. The CDC estimates that there are 300-500 million cases of malaria each year, and more than 1 million people die from it. It presents a major disease hazard for travelers to warm climates.
In some areas of the world, mosquitoes that carry malaria have developed resistance to insecticides. In addition, the parasites have developed resistance to some antibiotics. These conditions have led to difficulty in controlling both the rate of infection and spread of this disease.
There are four types of common malaria parasites. Recently, a fifth type, Plasmodium knowlesi. has been causing malaria in Malaysia and areas of southeast Asia. Another type, falciparum malaria, affects more red blood cells than the other types and is much more serious. It can be fatal within a few hours of the first symptoms.
Back to Top Symptoms
Back to Top Exams and Tests
During a physical examination, the doctor may find an enlarged liver or enlarged spleen. Malaria blood smears taken at 6 to 12 hour intervals confirm the diagnosis.
A complete blood count (CBC) will identify anemia if it is present.
Back to Top Treatment
Malaria, especially Falciparum malaria, is a medical emergency that requires a hospital stay. Chloroquine is often used as an anti-malarial medication. However, chloroquine-resistant infections are common in some parts of the world.
Possible treatments for chloroquine-resistant infections include:
- The combination of quinidine or quinine plus doxycycline, tetracycline, or clindamycin
- Atovaquone plus proguanil (Malarone)
- Mefloquine or artesunate
- The combination of pyrimethamine and sulfadoxine (Fansidar)
The choice of medication depends in part on where you were when you were infected.
Medical care, including fluids through a vein (IV) and other medications and breathing (respiratory) support may be needed.
Back to Top Outlook (Prognosis)
The outcome is expected to be good in most cases of malaria with treatment, but poor in Falciparum infection with complications.
Back to Top Possible Complications
- Brain infection (cerebritis)
- Destruction of blood cells (hemolytic anemia )
- Kidney failure
- Liver failure
- Meningitis
- Respiratory failure from fluid in the lungs (pulmonary edema )
- Rupture of the spleen leading to massive internal bleeding (hemorrhage)
Back to Top When to Contact a Medical Professional
Call your health care provider if you develop fever and headache after visiting the tropics.
Back to Top Prevention
Most people who live in areas where malaria is common have gotten some immunity to the disease. Visitors will not have immunity, and should take preventive medications.
It is important to see your health care provider well before your trip, because treatment may need to begin as long as 2 weeks before travel to the area, and continue for a month after you leave the area. In 2006, the CDC reported that most travelers from the U.S. who contracted malaria failed to take the right precautions.
The types of anti-malarial medications prescribed will depend on the area you visit. According to the CDC, travelers to South America, Africa, the Indian subcontinent, Asia, and the South Pacific should take one of the following drugs: mefloquine, doxycycline, chloroquine, hydroxychloroquine, or Malarone. Even pregnant women should take preventive medications because the risk to the fetus from the medication is less than the risk of catching this infection.
People who are taking anti-malarial medications may still become infected. Avoid mosquito bites by wearing protective clothing over the arms and legs, using screens on windows, and using insect repellent.
Chloroquine has been the drug of choice for protecting against malaria. But because of resistance, it is now only suggested for use in areas where Plasmodium vivax. P. oval. and P. malariae are present. Falciparum malaria is becoming increasingly resistant to anti-malarial medications.
For travelers going to areas where Falciparum malaria is known to occur, there are several options for malaria prevention, including mefloquine, atovaquone/proguanil (Malarone), and doxycycline.
Travelers can call the CDC for information on types of malaria in a certain area, preventive drugs, and times of the year to avoid travel. See: www.cdc.gov
Back to Top References
Fairhurst RM, Wellems TE. Plasmodium species (Malaria). In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, Pa: Elsevier Churchill Livingstone; 2009:chap 275.
Krogstad DJ. Malaria. In: Goldman L, Ausiello D, eds. Cecil Medicine. 23rd ed. Philadelphia, Pa: Saunders Elsevier. 2007:chap 366.
More Information on This Topic
Review Date: 6/9/2011
Reviewed By: David C. Dugdale, III, MD, Professor of Medicine, Division of General Medicine, Department of Medicine, University of Washington School of Medicine; Jatin M. Vyas, MD, PhD, Assistant Professor in Medicine, Harvard Medical School, Assistant in Medicine, Division of Infectious Disease, Department of Medicine, Massachusetts General Hospital. Also reviewed by David Zieve, MD, MHA, Medical Director, A.D.A.M. Inc.
A.D.A.M. Inc. is accredited by URAC, also known as the American Accreditation HealthCare Commission (www.urac.org). URAC’s accreditation program is an independent audit to verify that A.D.A.M. follows rigorous standards of quality and accountability. A.D.A.M. is among the first to achieve this important distinction for online health information and services. Learn more about A.D.A.M.’s editorial policy, editorial process and privacy policy. A.D.A.M. is also a founding member of Hi-Ethics and subscribes to the principles of the Health on the Net Foundation (www.hon.ch).
A.D.A.M. Copyright
The information provided herein should not be used during any medical emergency or for the diagnosis or treatment of any medical condition. A licensed medical professional should be consulted for diagnosis and treatment of any and all medical conditions. Call 911 for all medical emergencies. Links to other sites are provided for information only — they do not constitute endorsements of those other sites. © 1997- 2008 A.D.A.M. Inc. Any duplication or distribution of the information contained herein is strictly prohibited.
Chapter 3 Infectious Diseases Related to Travel
Paul M. Arguin, Kathrine R. Tan
INFECTIOUS AGENT
Malaria in humans is caused by protozoan parasites of the genus Plasmodium. Plasmodium falciparum. P. vivax,P. ovale. or P. malariae. In addition, P. knowlesi. a parasite of Old World (Eastern Hemisphere) monkeys, has been documented as a cause of human infections and some deaths in Southeast Asia.
TRANSMISSION
All species are transmitted by the bite of an infective female Anopheles mosquito. Occasionally, transmission occurs by blood transfusion, organ transplantation, needle sharing, or congenitally from mother to fetus.
EPIDEMIOLOGY
Malaria is a major international public health problem, causing an estimated 207 million infections worldwide and 627,000 deaths in 2012, according to the World Health Organization (WHO) World Malaria report 2013. Although these numbers are decreasing, the numbers of cases of malaria in travelers has been increasing steadily for the past 4 years. Despite the apparent progress in reducing the global prevalence of malaria, many areas remain malaria endemic, and the use of prevention measures by travelers is still inadequate. Information about malaria transmission in specific countries (see Yellow Fever & Malaria Information, by Country later in this chapter) is derived from various sources, including WHO. The information presented herein was accurate at the time of publication; however, factors that can change rapidly and from year to year (such as local weather conditions, mosquito vector density, and prevalence of infection) can markedly affect local malaria transmission patterns. Updated information may be found on the CDC website at www.cdc.gov/malaria. Tools such as the Malaria Map Application can assist in locating more unusual destinations and determining if malaria transmission occurs there (www.cdc.gov/malaria/map ).
Malaria transmission occurs in large areas of Africa, Latin America, parts of the Caribbean, Asia (including South Asia, Southeast Asia, and the Middle East), Eastern Europe, and the South Pacific (Maps 3-09 and 3-10 ).
The risk for acquiring malaria differs substantially from traveler to traveler and from region to region, even within a single country. This variability is a function of the intensity of transmission within the various regions and the itinerary, duration, season, and type of travel. In 2012, almost 1,700 cases of malaria (including 6 deaths) diagnosed in the United States and its territories were reported to CDC. Of these, 79% were acquired in Africa, 13% in Asia, 7% in the Caribbean and the Americas, and <1% in Oceania. These absolute numbers of cases should be considered in the context of the volume of travel to these locations. Travelers with the highest estimated relative risk for infection are those going to West Africa and Oceania. Travelers going to other parts of Africa, South Asia, and South America have a moderate estimated relative risk for infection. Travelers with lower estimated relative risk are those going to Mexico, Central America, and other parts of Asia.
Map 3-09. Malaria-endemic countries in the Western Hemisphere 1
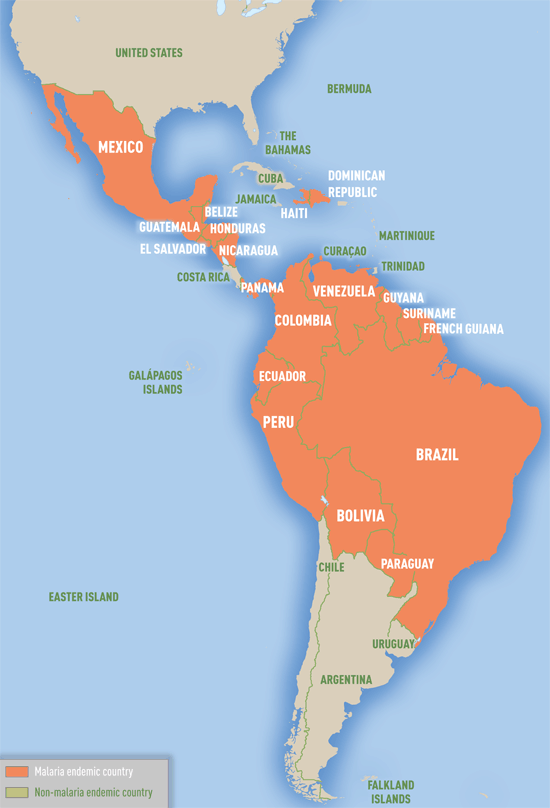
1 In this map, countries with areas endemic for malaria are shaded completely even if transmission occurs only in a small part of the country. For more specific within-country malaria transmission information, see the Yellow Fever & Malaria Information, by Country section in this chapter.
Map 3-10. Malaria-endemic countries in the eastern hemisphere 1

1 In this map, countries with areas endemic for malaria are shaded completely even if transmission occurs only in a small part of the country. For more specific within-country malaria transmission information, see the Yellow Fever & Malaria Information, by Country section in this chapter.
CLINICAL PRESENTATION
Malaria is characterized by fever and influenzalike symptoms, including chills, headache, myalgias, and malaise; these symptoms can occur at intervals. Uncomplicated disease may be associated with anemia and jaundice. In severe disease, seizures, mental confusion, kidney failure, acute respiratory distress syndrome, coma, and death may occur. Malaria symptoms can develop as early as 7 days (usually ≥14 days) after initial exposure in a malaria-endemic area and as late as several months or more after departure. Suspected or confirmed malaria, especially P. falciparum. is a medical emergency, requiring urgent intervention as clinical deterioration can occur rapidly and unpredictably. See Box 3-2 detailing some clinical highlights for malaria.
Box 3-02. Clinical highlights for malaria
- Overdose of antimalarial drugs, particularly chloroquine, can be fatal. Medication should be stored in childproof containers out of the reach of infants and children.
- Chemoprophylaxis can be started earlier if there are particular concerns about tolerating a medication. For example, mefloquine can be started 3–4 weeks in advance to allow potential adverse events to occur before travel. If unacceptable side effects develop, there would be time to change the medication before the traveler’s departure.
- The drugs used for antimalarial chemoprophylaxis are generally well tolerated. However, side effects can occur. Minor side effects usually do not require stopping the drug. Travelers who have serious side effects should see a clinician who can determine if their symptoms are related to the medicine and make a medication change.
- In comparison with drugs with short half-lives, which are taken daily, drugs with longer half-lives, which are taken weekly, offer the advantage of a wider margin of error if the traveler is late with a dose. For example, if a traveler is 1–2 days late with a weekly drug, prophylactic blood levels can remain adequate; if the traveler is 1–2 days late with a daily drug, protective blood levels are less likely to be maintained.
- In those who are glucose-6-phosphate dehydrogenase (G6PD) deficient, primaquine can cause hemolysis, which can be fatal. Be sure to document a normal G6PD level before prescribing primaquine.
- Travelers should be informed that malaria could be fatal if treatment is delayed. Medical help should be sought promptly if malaria is suspected, and a blood sample should be taken and examined for malaria parasites on 1 or more occasions.
- Travelers should also be informed that malaria could be fatal even when treated, which is why it is always preferable to prevent malaria cases rather than rely on treating infections after they occur.
- Malaria smear results or a rapid diagnostic test must be available immediately (within a few hours). Sending specimens to offsite laboratories where results are not available for extended periods of time (days) is not acceptable. If a patient has an illness suggestive of severe malaria and a compatible travel history in an area where malaria transmission occurs, it is advisable to start treatment as soon as possible, even before the diagnosis is established. CDC recommendations for malaria treatment can be found at www.cdc.gov/malaria/diagnosis_treatment/index.html .
DIAGNOSIS
Travelers who have symptoms of malaria should seek medical evaluation as soon as possible. Clinicians should consider malaria in any patient with a febrile illness who has recently returned from a malaria-endemic country.
Smear microscopy remains the gold standard for malaria diagnosis. Microscopy can also be used to determine the species of malaria parasite, identify the parasite life-cycle stages present, and quantify the parasitemia—all of which are necessary for providing the most appropriate treatment. Microscopy results should ideally be available within a few hours. It is an unacceptable practice to send these tests to an offsite laboratory or batch them for results to be provided days later.
Various test kits are available to detect antigens derived from malaria parasites. Such immunologic (immunochromatographic) tests most often use a dipstick or cassette format and provide results in 2–15 minutes. These rapid diagnostic tests (RDTs) offer a useful alternative to microscopy in situations where reliable microscopic diagnosis is not immediately available. Although RDTs can detect malaria antigens within minutes, they cannot determine the species, are less sensitive for diagnosis, and cannot quantify parasitemia. In addition, CDC recommends that positive and negative results always be confirmed by microscopy. Although confirmation does not have to occur simultaneously with the RDT, the information from microscopy, including the actual presence of malaria parasites, the species, life-cycle stages (asexual vs sexual blood-stage forms), and parasitemia will be most useful if it is available as soon as possible. The Food and Drug Administration (FDA) has approved 1 RDT for use in the United States by hospital and commercial laboratories, not by individual clinicians or by patients themselves. This RDT is called BinaxNOW Malaria test. Laboratories that do not provide in-house on-the-spot microscopy services should maintain a stock of malaria RDTs so that they will be able to perform malaria diagnostic testing when needed.
PCR tests are also available for detecting malaria parasites. Although these tests are more sensitive than routine microscopy, results are not usually available as quickly as microscopy results should be, thus limiting the utility of this test for acute diagnosis. PCR testing is most useful for definitively identifying the species of malaria parasite and detecting mixed infections. Species confirmation by PCR is available at the CDC malaria laboratory.
In sub-Saharan Africa, clinical overdiagnosis and the rate of false-positive microscopy for malaria may be high. Travelers to this region should be warned they may be diagnosed with malaria incorrectly, even though they are taking a reliable antimalarial regimen. In such cases, acutely ill travelers should be advised to seek the best available medical services and follow the treatment offered locally (except the use of halofantrine, which is not recommended; see below) but not to stop their chemoprophylaxis regimen.
TREATMENT
Malaria can be treated effectively early in the course of the disease, but delay of therapy can have serious or even fatal consequences. Travelers who have symptoms of malaria should be advised to seek medical evaluation as soon as possible. Specific treatment options depend on the species of malaria, the likelihood of drug resistance (based on where the infection was acquired), the age of the patient, pregnancy status, and the severity of infection.
Detailed CDC recommendations for malaria treatment can be found at www.cdc.gov/malaria/diagnosis_treatment/treatment.html. Clinicians who require assistance with the diagnosis or treatment of malaria should call the CDC Malaria Hotline (770-488-7788 or toll-free at 855-856-4713) from 9 am to 5 pm Eastern Time. After hours or on weekends and holidays, clinicians requiring assistance should call the CDC Emergency Operations Center at 770-488-7100 and ask the operator to page the person on call for the Malaria Branch. In addition, it is advisable to consult with a clinician who has specialized travel or tropical medicine expertise or with an infectious disease physician.
Medications that are not used in the United States for the treatment of malaria, such as halofantrine, are widely available overseas. CDC does not recommend halofantrine for treatment because of cardiac adverse events, including deaths, which have been documented after treatment. These adverse events have occurred in people with and without preexisting cardiac problems and both in the presence and absence of other antimalarial drugs (such as mefloquine).
Travelers who reject the advice to take prophylaxis, who choose a suboptimal drug regimen (such as chloroquine in an area with chloroquine-resistant P. falciparum ), or who require a less-than-optimal drug regimen for medical reasons are at increased risk for acquiring malaria and needing prompt treatment while overseas. In addition, some travelers who are taking effective prophylaxis but who will be in remote areas may decide, in consultation with their travel health provider, to take along a reliable supply of a full course of an approved malaria treatment regimen (see Box 3-03 for a definition of reliable supply ). In the event that they are diagnosed with malaria, they will have immediate access to this treatment regimen, which if acquired in the United States is unlikely to be counterfeit and will not deplete local resources. In rare instances when access to medical care is not available and the traveler develops a febrile illness consistent with malaria, the reliable supply medication can be self-administered presumptively. Travelers should be advised that this self-treatment of a possible malarial infection is only a temporary measure and that prompt medical evaluation is imperative.
Two malaria treatment regimens available in the United States can be prescribed as a reliable supply: atovaquone-proguanil and artemether-lumefantrine. The use of the same or related drugs that have been taken for prophylaxis is not recommended to treat malaria. For example, atovaquone-proguanil may be used as a reliable supply medication by travelers not taking atovaquone-proguanil for prophylaxis. See Table 3-08 for the dosing recommendation.
Box 3-03. What is a reliable supply?
ATOVAQUONE-PROGUANIL
The adult tablet contains 250 mg atovaquone and 100 mg proguanil. The pediatric tablet contains 62.5 mg atovaquone and 25 mg proguanil.
4 adult tablets, orally as a single daily dose for 3 consecutive days
Daily dose to be taken for 3 consecutive days:
5–8 kg: 2 pediatric tablets
9–10 kg: 3 pediatric tablets
11–20 kg: 1 adult tablet
21–30 kg: 2 adult tablets
31–40 kg: 3 adult tablets
>41 kg: 4 adult tablets
Contraindicated in people with severe renal impairment (creatinine clearance <30 mL/min)
Not recommended for people on atovaquone-proguanil prophylaxis
Not recommended for children weighing <5 kg, pregnant women, and women breastfeeding infants weighing <5 kg
ARTEMETHER-LUMEFANTRINE
One tablet contains 20 mg artemether and 120 mg lumefantrine.
A 3-day treatment schedule with a total of 6 oral doses is recommended for both adult and pediatric patients based on weight. The patient should receive the initial dose, followed by the second dose 8 hours later, then 1 dose twice per day for the following 2 days.
5 to <15 kg: 1 tablet per dose
15 to <25 kg: 2 tablets per dose
25 to <35 kg: 3 tablets per dose
≥35 kg: 4 tablets per dose
Not for people on mefloquine prophylaxis
Not recommended for children weighing <5 kg, pregnant women, and women breastfeeding infants weighing <5 kg
1 If used for presumptive self-treatment, medical care should be sought as soon as possible.
PREVENTION
The goal of these malaria prevention guidelines is to prevent malaria caused by all species, not only P. falciparum. These guidelines apply to both short-term and long-term travelers. Malaria prevention consists of a combination of mosquito avoidance measures and chemoprophylaxis. Although efficacious, the recommended interventions are not 100% effective. For details about how CDC determines malaria prevention recommendations for travelers, see Box 3-04 .
Preventing malaria involves striking a balance between ensuring that all people at risk for infection use the recommended prevention measures, while preventing rare occurrences of adverse effects of these interventions among people using them unnecessarily. An individual risk assessment should be conducted for every traveler, taking into account not only the destination country but also the detailed itinerary, including specific cities, types of accommodation, season, and style of travel. In addition, conditions such as pregnancy or antimalarial drug resistance at the destination may modify the risk assessment.
Depending on the level of risk, it may be appropriate to recommend no specific interventions, mosquito avoidance measures only, or mosquito avoidance measures plus chemoprophylaxis. For areas of intense transmission, such as West Africa, exposure for even short periods of time can result in transmission, so travelers to this area should be considered at high risk for infection. Malaria transmission is not distributed homogeneously throughout all countries. Some destinations have malaria transmission occurring only in certain areas. If travelers are going to high-transmission areas during peak transmission times, even though the country as a whole may have relatively low malaria transmission, they may be at high risk for infection while there.
Geography is just a part of determining a traveler’s risk for infection. Risk can differ substantially for different travelers as their behaviors and circumstances differ. For example, travelers staying in air-conditioned hotels may be at lower risk than backpackers or adventure travelers. Similarly, long-term residents living in screened and air-conditioned housing are less likely to be exposed than are people living without such amenities. The highest risk is associated with first- and second-generation immigrants living in non-endemic countries who return to their countries of origin to visit friends and relatives (VFRs). VFR travelers often consider themselves to be at no risk, because they grew up in a malarious country and consider themselves immune. However, acquired immunity is lost quickly, and VFRs should be considered to have the same risk as other nonimmune travelers (see Chapter 8, Immigrants Returning Home to Visit Friends & Relatives (VFRs) ). Travelers should also be reminded that even if a person has had malaria before, he or she can get it again, and preventive measures are still necessary.
Mosquito Avoidance Measures
Because of the nocturnal feeding habits of Anopheles mosquitoes, malaria transmission occurs primarily between dusk and dawn. Contact with mosquitoes can be reduced by remaining in well-screened areas, using mosquito bed nets (preferably insecticide-treated nets), using an effective insecticide spray in living and sleeping areas during evening and nighttime hours, and wearing clothes that cover most of the body.
All travelers should use an effective mosquito repellent (see Chapter 2, Protection against Mosquitoes, Ticks, & Other Arthropods ). Repellents should be applied to exposed parts of the skin when mosquitoes are likely to be present. If travelers are also wearing sunscreen, sunscreen should be applied first and insect repellent second. In addition to using a topical insect repellent, a permethrin-containing product may be applied to bed nets and clothing for additional protection against mosquitoes.
Chemoprophylaxis
All recommended primary chemoprophylaxis regimens involve taking a medicine before, during, and after travel to an area with malaria. Beginning the drug before travel allows the antimalarial agent to be in the blood before the traveler is exposed to malaria parasites. In choosing a chemoprophylaxis regimen before travel, the traveler and the travel health provider should consider several factors. The travel itinerary should be reviewed in detail and compared with information on where malaria transmission occurs within a given country, to determine whether the traveler will be traveling in a part of the country where malaria occurs and if antimalarial drug resistance has been reported in that location (see the Yellow Fever & Malaria Information, by Country section later in this chapter). Additional factors to consider are the patient’s other medical conditions, medications being taken (to assess potential drug interactions), the cost of the medicines, and the potential side effects. Table 3-09 lists some of the benefits and limitations of medicines used for malaria chemoprophylaxis; additional information about choosing a malaria chemoprophylaxis regimen can be found at www.cdc.gov/malaria/travelers/drugs.html .
The resistance of P. falciparum to chloroquine has been confirmed in all areas with P. falciparum malaria except the Caribbean, Central America west of the Panama Canal, and some countries in the Middle East. In addition, resistance to sulfadoxine-pyrimethamine is widespread in the Amazon River Basin area of South America, much of Southeast Asia, other parts of Asia, and in large parts of Africa. Resistance to mefloquine has been confirmed on the borders of Thailand with Burma (Myanmar) and Cambodia, in the western provinces of Cambodia, in the eastern states of Burma on the border between Burma and China, along the borders of Laos and Burma, the adjacent parts of the Thailand–Cambodia border, and in southern Vietnam. The resistance of P. vivax to chloroquine has been confirmed in Papua New Guinea and Indonesia.
In addition to primary prophylaxis, presumptive antirelapse therapy (also known as terminal prophylaxis) uses a medication toward the end of the exposure period (or immediately thereafter) to prevent relapses or delayed-onset clinical presentations of malaria caused by hypnozoites (dormant liver stages) of P. vivax or P. ovale. Because most malarious areas of the world (except the Caribbean) have at least 1 species of relapsing malaria, travelers to these areas have some risk for acquiring either P. vivax or P. ovale. although the actual risk for an individual traveler is difficult to define. Presumptive antirelapse therapy is generally indicated only for people who have had prolonged exposure in malaria-endemic areas (such as missionaries, military personnel, or Peace Corps volunteers).
The medications recommended for chemoprophylaxis of malaria may also be available at overseas destinations. However, combinations of these medications and additional drugs that are not recommended may be commonly prescribed and used in other countries. Travelers should be strongly discouraged from obtaining chemoprophylaxis medications while abroad. The quality of these products is not known; they may not be protective and could be dangerous. These medications may have been produced by substandard manufacturing practices, may be counterfeit, or may contain contaminants. Additional information on this topic can be found in Chapter 2, Perspectives: Pharmaceutical Quality & Counterfeit Drugs . and on the FDA website (www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/BuyingMedicinefromOutsidetheUnitedStates/default.htm ).
Medications Used for Chemoprophylaxis
Atovaquone-Proguanil
Atovaquone-proguanil is a fixed combination of the drugs atovaquone and proguanil. Prophylaxis should begin 1–2 days before travel to malarious areas and should be taken daily, at the same time each day, while in the malarious areas, and daily for 7 days after leaving the areas (see Table 3-10 for recommended dosages). Atovaquone-proguanil is well tolerated, and side effects are rare. The most common adverse effects reported in people using atovaquone-proguanil for prophylaxis or treatment are abdominal pain, nausea, vomiting, and headache. Atovaquone-proguanil is not recommended for prophylaxis in children weighing <5 kg (11 lb), pregnant women, or patients with severe renal impairment (creatinine clearance <30 mL/min). Proguanil may increase the effect of warfarin, so international normalized ratio monitoring or dosage adjustment may be needed.
Chloroquine and Hydroxychloroquine
Chloroquine phosphate or hydroxychloroquine sulfate can be used for prevention of malaria only in destinations where chloroquine resistance is not present (see Maps 3-09 and 3-10 and the Yellow Fever & Malaria Information, by Country section later in this chapter). Prophylaxis should begin 1–2 weeks before travel to malarious areas. It should be continued by taking the drug once a week, on the same day of the week, during travel in malarious areas and for 4 weeks after a traveler leaves these areas (see Table 3-10 for recommended dosages). Reported side effects include gastrointestinal disturbance, headache, dizziness, blurred vision, insomnia, and pruritus, but generally these effects do not require that the drug be discontinued. High doses of chloroquine, such as those used to treat rheumatoid arthritis, have been associated with retinopathy; this serious side effect appears to be extremely unlikely when chloroquine is used for routine weekly malaria prophylaxis. Chloroquine and related compounds have been reported to exacerbate psoriasis. People who experience uncomfortable side effects after taking chloroquine may tolerate the drug better by taking it with meals. As an alternative, the related compound hydroxychloroquine sulfate may be better tolerated.
Doxycycline
Doxycycline prophylaxis should begin 1–2 days before travel to malarious areas. It should be continued once a day, at the same time each day, during travel in malarious areas and daily for 4 weeks after the traveler leaves such areas. Insufficient data exist on the antimalarial prophylactic efficacy of related compounds such as minocycline (commonly prescribed for the treatment of acne). People on a long-term regimen of minocycline who need malaria prophylaxis should stop taking minocycline 1–2 days before travel and start doxycycline instead. Minocycline can be restarted after the full course of doxycycline is completed (see Table 3-10 for recommended dosages).
Doxycycline can cause photosensitivity, usually manifested as an exaggerated sunburn reaction. The risk for such a reaction can be minimized by avoiding prolonged, direct exposure to the sun and by using sunscreen. In addition, doxycycline use is associated with an increased frequency of vaginal yeast infections. Gastrointestinal side effects (nausea or vomiting) may be minimized by taking the drug with a meal or by specifically prescribing doxycycline monohydrate or the enteric-coated doxycycline hyclate, rather than the generic doxycycline hyclate, which is often less expensive. To reduce the risk for esophagitis, travelers should be advised to swallow the medicine with sufficient fluids and not to take doxycycline before going to bed. Doxycycline is contraindicated in people with an allergy to tetracyclines, during pregnancy, and in infants and children aged <8 years. Vaccination with the oral typhoid vaccine Ty21a should be delayed for ≥24 hours after taking a dose of doxycycline.
Mefloquine
Mefloquine prophylaxis should begin ≥2 weeks before travel to malarious areas. It should be continued once a week, on the same day of the week, during travel in malarious areas and for 4 weeks after a traveler leaves such areas (see Table 3-10 for recommended dosages). Mefloquine has been associated with rare but serious adverse reactions (such as psychoses or seizures) at prophylactic doses; these reactions are more frequent with the higher doses used for treatment. Other side effects that have occurred in chemoprophylaxis studies include gastrointestinal disturbance, headache, insomnia, abnormal dreams, visual disturbances, depression, anxiety disorder, and dizziness. Other more severe neuropsychiatric disorders occasionally reported during postmarketing surveillance include sensory and motor neuropathies (including paresthesia, tremor, and ataxia), agitation or restlessness, mood changes, panic attacks, forgetfulness, confusion, hallucinations, aggression, paranoia, and encephalopathy. On occasion, psychiatric symptoms have been reported to continue long after mefloquine has been stopped. FDA also includes a boxed warning about rare reports of persistent dizziness after mefloquine use. Mefloquine is contraindicated for use by travelers with a known hypersensitivity to mefloquine or related compounds (such as quinine or quinidine) and in people with active depression, a recent history of depression, generalized anxiety disorder, psychosis, schizophrenia, other major psychiatric disorders, or seizures. It should be used with caution in people with psychiatric disturbances or a history of depression. A review of available data suggests that mefloquine may be used safely in people concurrently on β-blockers, if they have no underlying arrhythmia. However, mefloquine is not recommended for people with cardiac conduction abnormalities. Any traveler receiving a prescription for mefloquine must also receive a copy of the FDA medication guide, which can be found at www.accessdata.fda.gov/drugsatfda_docs/label/2008/019591s023lbl.pdf .
Primaquine
Primaquine phosphate has 2 distinct uses for malaria prevention: primary prophylaxis in areas with primarily P. vivax and presumptive antirelapse therapy (terminal prophylaxis).
When taken for primary prophylaxis, primaquine should be taken 1–2 days before travel to malarious areas, daily, at the same time each day, while in the malarious areas, and daily for 7 days after leaving the areas (see Table 3-10 for recommended dosages). Primary prophylaxis with primaquine obviates the need for presumptive antirelapse therapy.
When used for presumptive antirelapse therapy, primaquine is administered for 14 days after the traveler has left a malarious area. When chloroquine, doxycycline, or mefloquine is used for primary prophylaxis, primaquine is usually taken during the last 2 weeks of postexposure prophylaxis. When atovaquone-proguanil is used for prophylaxis, primaquine may be taken during the final 7 days of atovaquone-proguanil, and then for an additional 7 days. Primaquine should be given concurrently with the primary prophylaxis medication. However, if that is not feasible, the primaquine course should still be administered after the primary prophylaxis medication has been completed.
The most common adverse event in people with normal glucose-6-phosphate dehydrogenase (G6PD) levels is gastrointestinal upset if primaquine is taken on an empty stomach. This problem is minimized or eliminated if primaquine is taken with food. In G6PD-deficient people, primaquine can cause hemolysis that can be fatal. Before primaquine is used, G6PD deficiency MUST be ruled out by laboratory testing.
Travel to Areas with Limited Malaria Transmission
For destinations where malaria cases occur sporadically and risk for infection to travelers is assessed as being low, CDC recommends that travelers use mosquito avoidance measures only, and no chemoprophylaxis should be prescribed (see the Yellow Fever & Malaria Information, by Country section later in this chapter).
Travel to Areas with Mainly P. vivax Malaria
For destinations where the main species of malaria present is P. vivax. in addition to mosquito avoidance measures, primaquine is a good choice for primary prophylaxis for travelers who are not G6PD deficient. Its use for this indication is considered off-label in the United States. The predominant species of malaria and the recommended chemoprophylaxis medicines are listed in the Yellow Fever & Malaria Information, by Country section later in this chapter. For people unable to take primaquine, other drugs can be used as described below, depending on the presence of antimalarial drug resistance.
Travel to Areas with Chloroquine-Sensitive Malaria
For destinations where chloroquine-sensitive malaria is present, in addition to mosquito avoidance measures, the many effective chemoprophylaxis options include chloroquine, atovaquone-proguanil, doxycycline, mefloquine, and in some instances, primaquine for travelers who are not G6PD deficient. Longer-term travelers may prefer the convenience of weekly chloroquine, while shorter-term travelers may prefer the shorter course of atovaquone-proguanil or primaquine.
Travel to Areas with Chloroquine-Resistant Malaria
For destinations where chloroquine-resistant malaria is present, in addition to mosquito avoidance measures, chemoprophylaxis options are atovaquone-proguanil, doxycycline, and mefloquine.
Travel to Areas with Mefloquine-Resistant Malaria
For destinations where mefloquine-resistant malaria is present, in addition to mosquito avoidance measures, chemoprophylaxis options are either atovaquone-proguanil or doxycycline.
Chemoprophylaxis for Infants, Children, and Adolescents
Infants of any age or weight or children and adolescents of any age can contract malaria. Therefore, all children traveling to malaria-endemic areas should use the recommended prevention measures, which often include taking an antimalarial drug. In the United States, antimalarial drugs are available only in oral formulations and may taste bitter. Pediatric doses should be carefully calculated according to body weight but should never exceed adult dose. Pharmacists can pulverize tablets and prepare gelatin capsules for each measured dose. If the child is unable to swallow the capsules or tablets, parents should prepare the child’s dose of medication by breaking open the gelatin capsule and mixing the drug with a small amount of something sweet, such as applesauce, chocolate syrup, or jelly, to ensure the entire dose is delivered to the child. Giving the dose on a full stomach may minimize stomach upset and vomiting.
Chloroquine and mefloquine are options for use in infants and children of all ages and weights, depending on drug resistance at their destination. Primaquine can be used for children who are not G6PD deficient traveling to areas with principally P. vivax. Doxycycline may be used for children who are aged ≥8 years. Atovaquone-proguanil may be used for prophylaxis for infants and children weighing ≥5 kg (11 lb). Prophylactic dosing for children weighing <11 kg (24 lb) constitutes off-label use in the United States. Pediatric dosing regimens are contained in Table 3-10 .
Chemoprophylaxis during Pregnancy and Breastfeeding
Malaria infection in pregnant women can be more severe than in non-pregnant women. Malaria increases the risk for adverse pregnancy outcomes, including prematurity, spontaneous abortion, and stillbirth. For these reasons, and because no chemoprophylaxis regimen is completely effective, women who are pregnant or likely to become pregnant should be advised to avoid travel to areas with malaria transmission if possible (see Chapter 8, Pregnant Travelers ). If travel to a malarious area cannot be deferred, use of an effective chemoprophylaxis regimen is essential.
Pregnant women traveling to areas where chloroquine-resistant P. falciparum has not been reported may take chloroquine prophylaxis. Chloroquine has not been found to have any harmful effects on the fetus when used in the recommended doses for malaria prophylaxis; therefore, pregnancy is not a contraindication for malaria prophylaxis with chloroquine phosphate or hydroxychloroquine sulfate.
For travel to areas where chloroquine resistance is present, mefloquine is the only medication recommended for malaria chemoprophylaxis during pregnancy. In 2011, the FDA reviewed available data for mefloquine use during pregnancy and reclassified it from category C (animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks) to category B (animal reproduction studies have failed to demonstrate a risk to the fetus and there are no adequate and well-controlled studies in pregnant women).
Experts are investigating ways to examine the safety of atovaquone-proguanil use during pregnancy. Proguanil has been used for decades in pregnant women; however, until such time as these data are fully evaluated, atovaquone-proguanil is not recommended for use during pregnancy. Doxycycline is contraindicated for malaria prophylaxis during pregnancy because of the risk for adverse effects seen with tetracycline, a related drug, on the fetus, which include discoloration and dysplasia of the teeth and inhibition of bone growth. Primaquine should not be used during pregnancy because the drug may be passed transplacentally to a G6PD-deficient fetus and cause hemolytic anemia in utero.
Very small amounts of antimalarial drugs are excreted in the breast milk of lactating women. Because the quantity of antimalarial drugs transferred in breast milk is insufficient to provide adequate protection against malaria, infants who require chemoprophylaxis must receive the recommended dosages of antimalarial drugs listed in Table 3-10. Because chloroquine and mefloquine may be safely prescribed to infants, it is also safe for infants to be exposed to the small amounts excreted in breast milk. Although data are very limited about the use of doxycycline in lactating women, most experts consider the theoretical possibility of adverse events to the infant to be remote.
Although no information is available on the amount of primaquine that enters human breast milk, the mother and infant should be tested for G6PD deficiency before primaquine is given to a woman who is breastfeeding. Because data are not yet available on the safety of atovaquone-proguanil prophylaxis in infants weighing <5 kg (11 lb), CDC does not recommend it to prevent malaria in women breastfeeding infants weighing <5 kg. However, it can be used to treat women who are breastfeeding infants of any weight when the potential benefit outweighs the potential risk to the infant (such as treating a breastfeeding woman who has acquired P. falciparum malaria in an area of multidrug-resistant strains and who cannot tolerate other treatment options).
Choosing a Drug to Prevent Malaria
Recommendations for drugs to prevent malaria differ by country of travel and can be found in the Yellow Fever & Malaria Information, by Country section later in this chapter. Recommended drugs for each country are listed in alphabetical order and have comparable efficacy in that country. No antimalarial drug is 100% protective; therefore, prophylaxis must be combined with the use of personal protective measures (such as insect repellent, long sleeves, long pants, sleeping in a mosquito-free setting or using an insecticide-treated bed net). When several different drugs are recommended for an area, Table 3-09 may help in the decision-making process.
Changing Medications as a Result of Side Effects during Chemoprophylaxis
Medications recommended for prophylaxis against malaria have different modes of action that affect the parasites at different stages of the life cycle. Thus, if the medication needs to be changed because of side effects before a full course has been completed, there are some special considerations (see Table 3-11 ).
Box 3-04. How CDC arrives at recommendations for preventing malaria among travelers
Countries are not required to submit malaria surveillance data to CDC. On an ongoing basis, we actively solicit data from multiple sources, including World Health Organization (main and regional offices); national malaria control programs; international organizations, such as the International Society of Travel Medicine; CDC overseas staff; US military; academic, research, and aid organizations; and published records from the medical literature. We also make judgments about the reliability and accuracy of those data. When possible, we assess trends and consider the data in the context of what we know about the malaria control activities within that country or other mitigating factors, such as natural disasters, wars, and other events that may be affecting the ability to control malaria or accurately count and report it. We consider factors such as the volume of travel to that country and the number of acquired cases reported in the US surveillance system. Based on all those considerations, we then try to accurately describe areas of that country where transmission occurs, substantial occurrences of antimalarial drug resistance, the proportions of species present, and the recommended chemoprophylaxis options.
Table 3-09. Considerations when choosing a drug for malaria prophylaxis
REASONS TO CONSIDER USE OF THIS DRUG
REASONS TO CONSIDER AVOIDING USE OF THIS DRUG
- Good for last-minute travelers because the drug is started 1–2 days before travel.
- Some people prefer to take a daily medicine.
- Good choice for shorter trips because the traveler takes the medicine for only 7 days after traveling rather than 4 weeks.
- Well tolerated—side effects uncommon.
- Pediatric tablets are available and may be more convenient.
- Cannot be used by women who are pregnant or breastfeeding a child that weighs <5 kg.
- Cannot be taken by people with severe renal impairment.
- Tends to be more expensive than some of the other options (especially for long trips).
- Some people (including children) would rather not take a medicine every day.
- Some people would rather take medicine weekly.
- Good choice for long trips because it is taken only weekly.
- Some people are already taking hydroxychloroquine chronically for rheumatologic conditions; in those instances, they may not have to take an additional medicine.
- Can be used in all trimesters of pregnancy.
- Cannot be used in areas with chloroquine or mefloquine resistance.
- May exacerbate psoriasis.
- Some people would rather not take a weekly medication.
- For short trips, some people would rather not take medication for 4 weeks after travel.
- Not a good choice for last-minute travelers, because drug needs to be started 1–2 weeks before travel.
- Some people prefer to take a daily medicine.
- Good for last-minute travelers because the drug is started 1–2 days before travel.
- Tends to be the least expensive antimalarial.
- People who are already taking doxycycline chronically to prevent acne do not have to take an additional medicine.
- Doxycycline also can prevent some additional infections (such as rickettsial infections and leptospirosis), so it may be preferred by people planning to hike, camp, and swim in fresh water.
- Cannot be used by pregnant women and children aged <8 years.
- Some people would rather not take a medicine every day.
- For short trips, some people would rather not take medication for 4 weeks after travel.
- Women prone to getting vaginal yeast infections when taking antibiotics may prefer taking a different medicine.
- People may want to avoid the increased risk of sun sensitivity.
- Some people are concerned about the potential of getting an upset stomach from doxycycline.
- Some people would rather take medicine weekly
- Good choice for long trips because it is taken only weekly
- Can be used in all trimesters of pregnancy
- Cannot be used in areas with mefloquine resistance.
- Cannot be used in patients with certain psychiatric conditions.
- Cannot be used in patients with a seizure disorder.
- Not recommended for people with cardiac conduction abnormalities.
- Not a good choice for last-minute travelers because drug needs to be started ≥2 weeks before travel.
- Some people would rather not take a weekly medication.
- For short trips, some people would rather not take medication for 4 weeks after travel.
- It is the most effective medicine for preventing P. vivax. so it is a good choice for travel to places with >90% P. vivax .
- Good choice for shorter trips because you only have to take the medicine for 7 days after traveling rather than 4 weeks.
- Good for last-minute travelers because the drug is started 1–2 days before travel.
- Some people prefer to take a daily medicine.
- Cannot be used in patients with G6PD deficiency.
- Cannot be used in patients who have not been tested for G6PD deficiency.
- There are costs and delays associated with getting a G6PD test; however, it only has to be done once. Once a normal G6PD level is verified and documented, the test does not have to be repeated the next time primaquine is considered.
- Cannot be used by pregnant women.
- Cannot be used by women who are breastfeeding, unless the infant has also been tested for G6PD deficiency.
- Some people (including children) would rather not take a medicine every day.
- Some people are concerned about the potential of getting an upset stomach from primaquine.
Abbreviation: G6PD, glucose-6-phosphate dehydrogenase.
Adult tablets contain 250 mg atovaquone and 100 mg proguanil hydrochloride. 1 adult tablet orally, daily
Pediatric tablets contain 62.5 mg atovaquone and 25 mg proguanil hydrochloride.
5–8 kg: 1/2 pediatric tablet daily
>8–10 kg: 3/4 pediatric tablet daily
>10–20 kg: 1 pediatric tablet daily
>20–30 kg: 2 pediatric tablets daily
>30–40 kg: 3 pediatric tablets daily
>40 kg: 1 adult tablet daily
Begin 1–2 days before travel to malarious areas. Take daily at the same time each day while in the malarious area and for 7 days after leaving such areas. Contraindicated in people with severe renal impairment (creatinine clearance <30 mL/min). Atovaquone-proguanil should be taken with food or a milky drink. Not recommended for prophylaxis for children weighing <5 kg, pregnant women, and women breastfeeding infants weighing <5 kg. Partial tablet doses may need to be prepared by a pharmacist and dispensed in individual capsules, as described in the text.
Prophylaxis only in areas with chloroquine-sensitive malaria
300 mg base (500 mg salt) orally, once/week
5 mg/kg base (8.3 mg/kg salt) orally, once/week, up to maximum adult dose of 300 mg base
Begin 1–2 weeks before travel to malarious areas. Take weekly on the same day of the week while in the malarious area and for 4 weeks after leaving such areas. May exacerbate psoriasis.
Prophylaxis in all areas
100 mg orally, daily
≥8 years of age: 2.2 mg/kg up to adult dose of 100 mg/day
Begin 1–2 days before travel to malarious areas. Take daily at the same time each day while in the malarious area and for 4 weeks after leaving such areas. Contraindicated in children <8 years of age and pregnant women.
An alternative to chloroquine for prophylaxis only in areas with chloroquine-sensitive malaria
310 mg base (400 mg salt) orally, once/week
5 mg/kg base (6.5 mg/kg salt) orally, once/week, up to a maximum adult dose of 310 mg base
Begin 1–2 weeks before travel to malarious areas. Take weekly on the same day of the week while in the malarious area and for 4 weeks after leaving such areas.
Prophylaxis in areas with mefloquine-sensitive malaria
228 mg base (250 mg salt) orally, once/week
≤9 kg: 4.6 mg/kg base (5 mg/kg salt) orally, once/week
>9–19 kg: 1/4 tablet once/week
>19–30 kg: 1/2 tablet once/week
>30–45 kg: 3/4 tablet once/week
>45 kg: 1 tablet once/week
Begin ≥2 weeks before travel to malarious areas. Take weekly on the same day of the week while in the malarious area and for 4 weeks after leaving such areas. Contraindicated in people allergic to mefloquine or related compounds (quinine, quinidine) and in people with active depression, a recent history of depression, generalized anxiety disorder, psychosis, schizophrenia, other major psychiatric disorders, or seizures. Use with caution in persons with psychiatric disturbances or a previous history of depression. Not recommended for persons with cardiac conduction abnormalities.
Prophylaxis for short-
duration travel to areas with principally P.vivax
30 mg base (52.6 mg salt) orally, daily
0.5 mg/kg base (0.8 mg/kg salt) up to adult dose orally, daily
Begin 1–2 days before travel to malarious areas. Take daily at the same time each day while in the malarious area and for 7 days after leaving such areas. Contraindicated in people with G6PD deficiency. Also contraindicated during pregnancy and lactation, unless the infant being breastfed has a documented normal G6PD level.
Used for presumptive antirelapse therapy (terminal prophylaxis) to decrease the risk for relapses of P. vivax and P. ovale
30 mg base (52.6 mg salt) orally, daily for 14 days after departure from the malarious area
0.5 mg/kg base (0.8 mg/kg salt) up to adult dose orally, daily for 14 days after departure from the malarious area
Indicated for people who have had prolonged exposure to P. vivax. P. ovale. or both. Contraindicated in people with G6PD deficiency. Also contraindicated during pregnancy and lactation, unless the infant being breastfed has a documented normal G6PD level.
Abbreviation: G6PD, glucose-6-phosphate dehydrogenase.
1 All people who take primaquine should have a documented normal G6PD level before starting the medication.
Begin doxycycline, continue daily while in malaria-endemic area, and continue for 4 weeks after leaving malaria-endemic area.
- If the switch occurs ≥3 weeks before departure from the endemic area, atovaquone-proguanil should be taken daily for the remainder of the stay in the endemic area and for 1 week thereafter.
- If the switch occurs <3 weeks before departure from the endemic area, atovaquone-proguanil should be taken daily for 4 weeks after the switch.
- If the switch occurs after departure from the risk area, atovaquone-proguanil should be taken daily until 4 weeks after the date of departure.
This switch would be unlikely as primaquine is only recommended for primary prophylaxis in areas with mainly P. vivax for people with normal G6PD activity. Should that be the case, begin primaquine, continue daily while in malaria-endemic area, and continue for 7 days after leaving malaria-endemic area.
- If the switch occurs ≥3 weeks before departure from the endemic area, atovaquone-proguanil should be taken daily for the remainder of the stay in the endemic area and for 1 week thereafter.
- If the switch occurs <3 weeks before departure from the endemic area, atovaquone-proguanil should be taken daily for 4 weeks after the switch.
- If the switch occurs after departure from the risk area, atovaquone-proguanil should be taken daily until 4 weeks after the date of departure.
This switch would be unlikely as primaquine is only recommended for primary prophylaxis in areas with mainly P. vivax for people with normal G6PD activity. Should that be the case, begin primaquine, continue daily while in malaria-endemic area, and continue for 7 days after leaving malaria-endemic area.
Begin doxycycline, continue daily while in malaria-endemic area, and continue for 4 weeks after leaving malaria-endemic area.
This switch would be unlikely as primaquine is only recommended for primary prophylaxis in areas with mainly P. vivax for people with normal G6PD activity. Should that be the case, begin primaquine, continue daily while in malaria-endemic area, and continue for 7 days after leaving malaria-endemic area.
Begin doxycycline, continue daily while in malaria-endemic area, and continue for 4 weeks after leaving malaria-endemic area.
- If the switch occurs ≥3 weeks before departure from the endemic area, atovaquone-proguanil should be taken daily for the remainder of the stay in the endemic area and for 1 week thereafter.
- If the switch occurs <3 weeks before departure from the endemic area, atovaquone-proguanil should be taken daily for 4 weeks after the switch.
- If the switch occurs following departure from the risk area, atovaquone-proguanil should be taken daily until 4 weeks after the date of departure from the endemic area.
This switch would be unlikely as primaquine is only recommended for primary prophylaxis in areas with mainly P. vivax for people with normal G6PD activity. Should that be the case, begin primaquine, continue daily while in malaria-endemic area, and continue for 7 days after leaving malaria-endemic area.
Begin doxycycline, continue daily while in malaria-endemic area, and continue for 4 weeks after leaving malaria-endemic area.
Begin atovaquone-proguanil, continue daily while in malaria-endemic area, and continue for 7 days after leaving malaria-endemic area.
Extraordinary Experiences from mydays:
Unique Presents for unforgettable Moments
Providing magic moments which make people happy – this motto has made the experience broker mydays a leading expert for eventful presents since the year 2003.
With its huge choice of hundreds of extraordinary experiences all over Europe, mydays features an outstanding variety of unique gift ideas with a range from spectacular action to romantic wellness trips.
Being the originator and market leader for Germany, mydays has successfully expanded to Austria and Switzerland.
Select your country and discover the fascinating experience world of mydays yourself!
mydays in Europe:

Doxycycline Malaria Cost
Related Posts:
prescription drugs doxycycline hyclate
has the price of doxycycline gone up
azithromycin order doxycycline
doxycycline rezeptfrei kaufen
prescription drugs doxycycline hyclate
boots pharmacy doxycycline price
buy doxycycline chlamydia
doxycycline vibramycin price
buy doxycycline thailand
walmart doxycycline price


